Lung Cancer Answers is a website devoted to life issues for lung cancer patients and their families and is sponsored by Brad Cooper* of Cooper, Hart, Leggiero & Whitehead, PLLC. Cooper, Hart, Leggiero & Whitehead is located in The Woodlands, Texas (Greater Houston Area), handles cases nationwide with co-counsel in state of filing, and can be reached toll-free at 1-800-998-9729 for more information on lung cancer. Brad Cooper is not a medical doctor. The information on these pages is for the education of lung cancer patients and their families regarding potential medical and legal options. Patients are advised to consult with a medical doctor.
* Licensed by the Supreme Courts of Texas.
Targeted Therapy for Lung Cancer Patients
Targeted therapy is a type of treatment that uses drugs or other substances to identify and attack specific cancer cells. Targeted therapies usually cause less harm to normal cells than chemotherapy or radiation therapy do.
Targeted therapies can be used to treat certain non-small cell lung cancers (NSCLC). Currently, there are three types available, which include:
- Epidermal growth factor receptor (EGFR) inhibitor therapy: EGFRs are proteins found on the surface of certain cells, including cancer cells. Epidermal growth factor attaches to the EGFR on the surface of the cell and causes the cells to grow and divide. EGFR inhibitors block the receptor and stop the epidermal growth factor from attaching to the cancer cell. This stops the cancer cell from growing and dividing.
- Anaplastic lymphoma kinase (ALK) Inhibitors: ALK inhibitors target the cancer-causing alteration in the ALK gene. These drugs continue to be refined for the five percent of lung cancer patients who have an ALK gene alteration.
- Angiogenesis inhibitors: Angiogenesis inhibitors block the growth of new blood vessels to tumors (a process called tumor angiogenesis). A blood supply is necessary for tumors to grow beyond a certain size because blood provides the oxygen and nutrients that tumors need for continued growth. Treatments that interfere with angiogenesis may block tumor growth.
Targeting Biomarkers
Certain cases of non-small cell lung cancers respond better to targeted therapies based on specific gene mutations found in the tumor cells. Therefore, it is necessary for tumors to undergo biomarker testing in order to determine if any mutations are present. Depending on which particular mutation the tumor may harbor, the patient’s treatment can be matched with what will potentially offer the best response rates in that specific case.
Lung Cancer Tumor Testing
To have a tumor tested, there needs to be enough available tissue. This tissue may be obtained through procedures such as surgery, VATS (video-assisted thoracoscopic surgery), and CT-guided core needle biopsy. Tumor samples obtained in these ways should provide enough material for analysis. Tissue obtained through fine needle aspiration may not prove adequate. Simply put, the larger the sample, the more likelihood of confirming the presence of a mutation.
Many academic centers, as well as certified laboratories, offer tumor testing services. Additionally, fourteen medical facilities in the U.S. are participating in a federally funded study called the Lung Cancer Consortium Protocol, offering advanced lung cancer patients a free screening of their tumors for genetic mutations, some of which may be targets for treatment with existing or experimental therapies. Tumors will be tested at no cost to the participant, and if applicable, medical professionals will notify them of clinical trials that specifically target the mutation found in their tumors. A database will also be compiled so that as new therapies are developed, patients can be contacted and linked to new trials.
Which Gene Mutations in Lung Cancer Can be Treated with Targeted Therapy?
While a number of different mutations have been identified in lung cancer to date, effective targeted therapies have not yet been developed for all of them. At the present time, the three mutations most commonly tested for are the EGFR, ALK, and KRAS genes. Since a positive result for one of these mutations generally means that the tumor will test negative for the others, treatment can be focused on where the best results may be obtained. Following is a chart showing the breakdown of currently identified non-small cell lung cancer mutations.
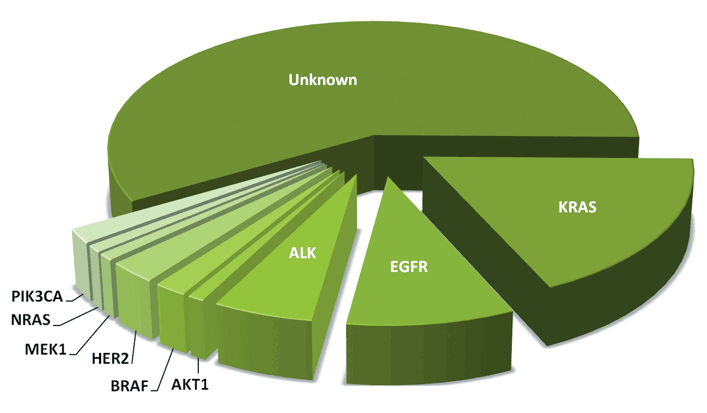
If you do not test positive for a biomarker with an approved targeted therapy, other methods of lung cancer treatment, such as surgery, chemotherapy, radiation, or immunotherapy may be recommended. Enrolling in a clinical trial looking at treatments for a number of other markers might also be an appropriate option.
EGFR Gene Mutations
EGFR-positive lung cancer represents about 10-15% of lung cancer in the United States and generally appears in adenocarcinoma, the most common subtype of non-small cell lung cancer. These mutations predominate in female never-smokers. Currently, the drugs of choice for targeted therapy of this mutation are erlotinib (Tarceva), gefitinib (Iressa), afatinib (Gilotrif), osimertinib (Tagrisso), and dacomitinib (Vizimpro). These drugs are classified as tyrosine kinase inhibitors and work by slowing or blocking the activity of a specific protein called Epidermal Growth Factor Receptor 1 (HER1/EGFR) which allows cancer cells to multiply. They are not chemotherapy drugs and are taken in pill form as prescribed by the doctor. While studies have shown these drugs to be successful in helping patients with the EGFR mutation to live longer, they appear to be somewhat limited by the ultimate development of resistance; approximately 50% eventually show the emergence of a second mutation. Second-generation EGFR inhibitors are in development, but efficacy has been limited due to toxicity.
ALK Gene Mutations
Approximately 3% to 7% of non-small cell lung cancers are ALK (anaplastic lymphoma kinase) mutations. Patients with ALK rearrangements are generally younger than most non-small cell lung cancer patients and are often never to light smokers. Recently, researchers have determined that a new drug, crizotinib, shows efficacy in patients with this mutation. Crizotinib (also known as Xalkori) is an FDA-approved drug used for advanced-stage NSCLC, which has been determined to be ALK (anaplastic lymphoma kinase) positive. It works by targeting the “driver kinase”, blocking its activity and preventing the tumor from growing. As with the EGFR inhibitors, however, tumors tend to adapt to target therapies, and eventually render them ineffective.
KRAS Gene Mutations
KRAS mutations are common in non-small cell lung cancer, and since a high percentage of those having this mutation have a smoking history, it has been hypothesized that there is a direct relationship to tobacco exposure. That said, not all studies are in complete agreement, and while it appears that some mutations in KRAS are associated with cigarette smoking, KRAS mutations also occur in never-smokers (less than 100 cigarettes per lifetime). Because of the commonality of this mutation, it has attracted considerable attention for targeted therapy. Unfortunately, however, KRAS inhibitors have proven clinically ineffective despite being tested in a large number of clinical trials. Research is being conducted on an ongoing basis.
Other Gene Mutations & Lung Cancer
Two other lung cancer mutations that occur with less frequency than EGFR and ALK, are the HER2 and BRAF mutations. Since some success has already been noted in other cancers (breast and melanoma) where these mutations occur, it would seem likely that similar success could be obtained with therapies targeting the same mutations in lung cancer.
HER2 Gene Mutations
HER2 is part of the same family of tyrosine kinase receptors as EGFR. In lung cancer, mutations in HER2 occur in only 2% to 3% of non-small cell lung cancers, and like EGFR and ALK, typically are seen in the adenocarcinoma subtype, and more frequently in women and never-smokers. Unfortunately, because of the relatively low percentage of patients with this particular mutation, routine tumor testing and the availability of clinical trials have been limited. At present, patients who are identified as HER2-mutant are treated with first-line chemotherapy, with HER2-specific trials designated as second-line or greater therapy. Although some patients have been able to obtain the drugs used to target HER2, (i.e., Herceptin and Tykerb) on an off-label basis, both drugs are expensive and there is difficulty in gaining approval for payment of treatments. The Lung Cancer Mutation Consortium is offering tumor testing for HER2 mutations with the goal of getting all participating medical institutions (currently fourteen) to join together to promote and complete clinical trials of this uncommon mutation.
BRAF Gene Mutations
Another target in non-small cell lung cancer is the BRAF mutation. While BRAF mutations are among the most common in cancers in general, they only represent 1% to 3% in lung cancer. They are most commonly found in adenocarcinomas and in former or current smokers.
Currently, the most promising studies of BRAF have been those associated with the treatment of melanoma, a potentially deadly form of skin cancer that has been notoriously difficult to treat. In contrast to BRAF mutations in melanoma, there appear to be many different BRAF mutations in lung cancer, making testing more complicated. Scientists are also unsure at this point which of these different mutations might respond best to targeted therapy. The Lung Cancer Mutation Consortium is testing for multiple BRAF mutations, with direction to clinical trials being offered through the Consortium.
Read more about:
If you, or someone you know, has lung cancer and you would like to know if they qualify for additional compensation, please call 1-800-998-9729 for a FREE consultation.

©2026 Cooper, Hart, Leggiero & Whitehead, PLLC. All rights reserved.